|
Grants:
2011 GRF (PI); 2012 ECS (PI); 2012 NSFC (PI); 2013 GRF (PI); 2014 GRF
(PI); 2015 Scheme-B (PI); 2016 BTF (PI); 2017 BTF (PI); 2018 GRF (PI);
2019 GRF (PI); 2020 GRF (PI)
Large group grants: 2012 TBR (co-I); 2014 973-grant (co-I); 2015 CRF
(co-I); 2017 TBR (co-I); 2018 TBR (Co-I); 2021 CRF (Co-PI); 2021 ITF
(Co-PI)
________________________________________________________________________________________________
Student
Awards:
2014 Reaching Out Award to HU Jiabiao
2016 Reaching Out Award to HE Xiangjun
2016 Postgraduate Research Output Award to HE Xiangjun
2017 SBS Postgraduate
Research Day 2017 The Third Prize to ZHANG Chenzi
2018 Third prize in
the oral presentation of 6th Cross-strait Symposium on Biomedical
Sciences at Kunming Institute of Zoology to ZHANG Chenzi
2019 2017-2018 Staff
Scholarship for BSc in Biomedical Sciences Programme to WANG Jingyi
2019 SBS Postgraduate
Research Day 2018 The Third Prize to ZHANG Chenzi
2019 SBS Postgraduate
Research Day 2018 The Top 10 Poster Award to ZHANG Chenzi
2021 Best Presentation -
Sliver Award in Poster Presentation 2021 to WONG Hoi Ting
2021 Best Presentation -
Sliver Award in Poster Presentation 2021 to LEUNG Sum Yin
2021 SBS
Postgraduate Research Day 2021 Second Prize in the Oral Presentation to
ZHANG Zhenjie
2021 SBS Postgraduate
Research Day 2021 Most Popular Poster Prize to ZHANG Zhenjie
2021 SBS Postgraduate
Research Day 2021 Most Popular Poster Prize to WANG Jingyi
2022 2020-2021
Staff Scholarship for BSc in Biomedical Sciences Programme to TSANG Kin
Ching
2022 Best
Presentation Award in Capstone Research Project Presentation Day 2022 to
TSANG Kin Ching
2022 Best Presentation –
Bronze Award in Poster Presentation to CHEUNG Lok Lam
2022 Prof. Leung Po Sing
Scholarship 2021/22 to WANG Jingyi
2022
“2022粵港再生醫學研究生學術交流”大會口頭報告二等獎 to ZHANG Siqi
2022
“2022粵港再生醫學研究生學術交流”大會墻報展示三等獎 to WEI Junkang
2022 CRMH Research Day
2022 – Best Oral Presentation Award to ZHANG Zhenjie
________________________________________________________________________________________________
Highlights of
Research:
1.
Knock-in of large
reporter genes in human cells via CRISPR/Cas9-induced homology-dependent
and independent DNA repair
CRISPR/Cas9-introduced
site-specific DNA double-strand breaks (DSBs) can be repaired by
homology-directed repair (HDR) or non-homologous end joining (NHEJ)
repair mechanisms. Extensive efforts have been made to knock-in exogenous
DNA to a selected genomic locus in human cells, including pluripotent
human embryonic stem cells (ESCs) and induced pluripotent stem cells
(iPSCs) which possess big potentials in regenerative medicine. However,
most of studies focused on HDR-based strategies which were proven
inefficient. We constructed a universal reporter system and
systematically investigate into the potentials of both HDR and NHEJ
repair in mediating CRISPR/Cas9-induced reporter integration. Here, we
found that NHEJ pathway mediates efficient rejoining
of genome and plasmids following CRISPR/Cas9-induced DNA
DSBs, and promotes high-efficiency DNA integration in various
human cell types. With this homology-independent knock-in strategy,
integration of a 4.6 kb promoterless ires-eGFP fragment into the GAPDH locus yielded up to
20% GFP+ cells in somatic LO2 cells, and 1.70% GFP+ cells in human
embryonic stem cells (ESCs). Quantitative comparison further demonstrated
that the NHEJ-based knock-in is more efficient than HDR-mediated gene
targeting in all human cell types examined. These data support that
CRISPR/Cas9-induced NHEJ provides a valuable new path for efficient
genome editing in human ESCs and somatic cells.

He et al., 2016, Nucleic Acids Research
2.
Developing safer
AAV-CRISPR gene knock-in therapy for the treatment of hemophilia
B in human
Recombinant
adenovirus associated virus (AAV) vector-based delivery of CRISPR/Cas9
(AAV-CRISPR) has shown promising potentials in preclinical models to
efficiently insert therapeutic gene sequences in somatic tissues.
However, the doses of AAV input required for effective targeting were
prohibitively high which posed serious risk of toxicity. We performed
AAV-CRISPR mediated homology independent knock-in at the proximal mAlb 3'UTR and demonstrated that single dose
of AAVs enabled long-term integration and expression of hF9
transgene in both adult and neonatal hemophilia
B mice (mF9 -/-), as evidenced by high levels of circulating hFIX and restored hemostasis
during the entire 48-week observation period. The germline genomes from
edited mice were free of modification. No evident changes at
transcriptome level were associated with AAV-CRISPR-mediated hF9
knock-in, and no off-target editing events were detected at the top 10 in
silico-predicted sites. Furthermore, we demonstrated that hemostasis correction can be efficiently achieved
with a much lower AAV dose (2 × 109 vg/neonate and 1.6 × 1010
vg/adult mouse) through liver-specific gene knock-in using hyperactive hF9R338L
variant. The serum antibodies against Cas9 and AAV in the neonatal mice
receiving low-dose AAV-CRISPR were negligible, which lent support to the
development of AAV-CRISPR mediated somatic knock-in for treating
inherited diseases.
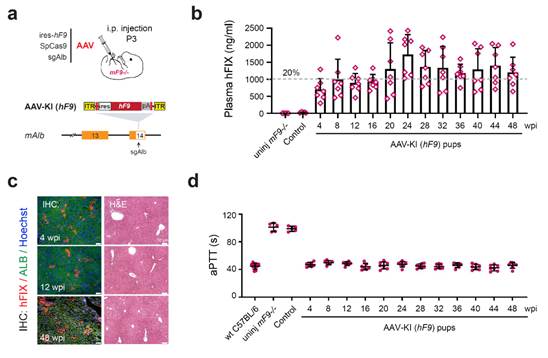
He
X. Zhang Z. et al., 2022, Nature Communications
3.
Generation of universal
CAR-T cells through one-step targeting
T-cells
engineered to express chimeric antigen receptor (CARs) represents an
innovative and revolutionizing approach for cancer immunotherapy. In the
clinic, CAR-T therapies targeting CD19 and BCMA have achieved overall
response rates (ORR) of 73% and 82%, respectively. However, the remaining
20-30% of patients still experienced relapsed or refractory (R/R)
diseases, which averted the eradication of malignant cells after CAR-T
therapy.
The
current FDA-approved CAR-T products are all based on autologous
transplantation, for which the manufacturing processes are exorbitantly
expensive, and varied quantity and quality of starting T-cells obtained
from patients significantly influence the treatment outcomes. To overcome
the inherent limitation of autologous CAR-T products, we have initiated a
research project to develop universal allogeneic CAR-T cells (UCAR-T) engineered
through AAV-delivered CRISPR/Cas9 gene editing to reduce the risk of
triggering GvHD (supported by ITF Ref. ITS/153/20FP). Our research has
yielded promising results in manufacturing large numbers of effective
U-CAR-T cells and demonstrated excellent efficacy against cancer and
tolerability using leukaemia xenograft mouse models.
The
development of “U-CAR-T” is anticipated to bypass the dependency on
autologous patient-specific T cells to broaden the range of patients to
receive treatment, particularly benefit pediatric
and heavily treated patients.
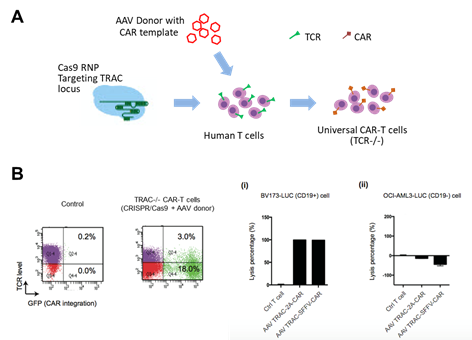
Zhang
C. Ngan CC. et al. unpublished
4.
Integrative view of
pluripotency through bioinformatics tools
Nowadays,
bioinformatics plays a crucial role in ESCs/iPSCs studies by providing
comprehensive interpretation through integrating the massive information
from multiple research platforms, such as microarray and next-generation
sequencing (NGS) data. In our lab, we use bioinformatics tools to
integrate the datasets of microarray, ChIP-seq,
RNA-seq, as well as methyl-seq. Intensive
knowledge can be acquired for understanding the underlying transcription
regulations, gene expressions, as well as epigenetic modifications during
stem cell differentiation and reprogramming processes. The research
output can provide valuable information to understand the molecular
mechanisms of pluripotent network in embryonic stem cells (Wang et al.
unpublished).
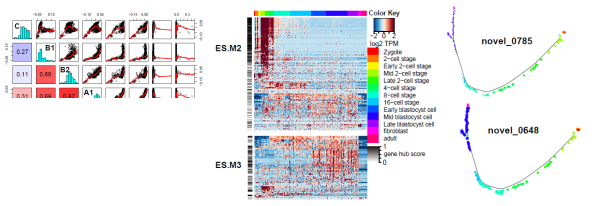
Wang
et al. unpublished
Projects in near future:
 AAV-CRISPR
strategies for gene and cell therapy AAV-CRISPR
strategies for gene and cell therapy
 Study of hepatic differentiation from
human ESC/iPSCs Study of hepatic differentiation from
human ESC/iPSCs
 Molecular mechanism underlying the
lineage specification and cell fate control Molecular mechanism underlying the
lineage specification and cell fate control
 Study on liver stem cells and their
applications in the treatment of end-stage liver disease Study on liver stem cells and their
applications in the treatment of end-stage liver disease
|